The desire to halt climate change has drawn researchers around the world to the pursuit of CO2 conversion.
Len Calderone for | AltEnergyMag
How do you power global transportation in the future in a way that is carbon neutral?
Can we pull energy from the atmosphere simply and at a low cost? Technology exists in the present day to extract carbon dioxide from the atmosphere to make fuel while making oxygen as a byproduct. By doing this, we have a desired energy source, while impacting the environment in a positive way.
Producing energy today requires the emission of carbon dioxide. To counter this, engineers have invented a process to produce liquid hydrocarbon fuels. Using concentrated light, heat and high pressure can produce a one-step conversion of carbon dioxide and water to useable liquid hydrocarbon fuel. The device used for the conversion is similar to a battery. It has two poles of charged material in a bath of chemical laced water.
Like a battery, the experimental device has two poles of charged materials resting in a bath of chemical-laced water. A small tube bubbles carbon dioxide into the device, called a cell. The CO2 interacts with the charged metal coating one of the poles and, with the help of a special catalyst, begins to form bigger molecules that combine carbon, hydrogen, and oxygen atoms—otherwise known as hydrocarbons—the molecules that make up the fuels that power the world. This method basically reverses combustion by taking CO2 and converts it back into a fuel that can be burned.
This process is not new. In the 1990s, a graduate student named Lin Chao at Princeton University decided to bubble carbon dioxide into an electrochemical cell. Using cathodes made from the element palladium and a catalyst known as pyridinium, he discovered that applying an electric current would extract methanol from the CO2. He published his findings in 1994—no one cared.
Today, research groups have been working to develop artificial photosynthesis, which could greatly reduce our dependence on crude oil and make use of the growing amount of manmade carbon dioxide emissions that contribute to climate change.
Artificial photosynthesis works takes solar energy to split water and carbon dioxide into hydrogen, oxygen and carbon. A catalyst then recombines the molecules to create liquid fuels, such as methanol. Methanol is the simplest hydrocarbon that works in internal combustion engines. China has already blended methanol into gasoline at levels of 15% or less, while taxi and bus fleets run on high-level blends of 85% methanol.
.jpg)
Researchers are also pursuing low-temperature electrolysis of CO2 and H2O with energy directly from sunlight. They are using light-absorbing semiconductors, such as titanium dioxide to churn out CO, methane, or other hydrocarbons. So far, such methods aren’t very efficient, converting less than 1% of the solar energy into chemical bonds. One research team has developed a bismuth-based photocatalyst that converts 6.1% of incoming visible light energy to chemical bonds in CO.
A start-up company, Carbon Engineering, which is partially funded by Bill Gates, built an air capture system, which will essentially do the job of trees, but in places where the land cannot be cultivated and there are no trees, such as icy plains and deserts.

Carbon Engineering is one of a handful of companies around the world that are now working on new ways to pull enough carbon dioxide out of the atmosphere to actually put a dent in the effects of climate change. Since CO2 is everywhere, air capture systems can be employed just about anywhere.
Captured liquid is pumped to the top of the air contractor and descends through corrugated sheets, while large fans push air through corrugated PVC sheets. The CO2 in the air is captured as it comes into contact with the liquid. Air that contains less CO2 exits through the back side of the corrugated sheets. The liquid is collected in a large tray and funneled into a sump, after which the liquid with captured CO2 is sent to a central regeneration facility. The regeneration facility extracts pure CO2 from the captured liquid and returns it to the sump. The CO2 is then combined with H2 to make hydrocarbon fuel.
Scientists are also working on a one-step process to covert water and CO2 into fuel, using concentrated light, heat and high pressure. The process is accomplished with a photo thermo chemical flow reactor operating at about 350 - 400° F and pressures up to 6 atmospheres.
.jpg)
Concentrated light drives the photochemical reaction, which generates high-energy intermediates and heat to drive thermo chemical carbon chain forming reactions, producing hydrocarbons in a single step process.
Nanotechnology can also help capture carbon dioxide created during industrial processes and convert it to fuel such as methane. This process not only keeps CO2 out of the air supply, but also turns it into a source of energy for the same factory.
Captured CO2 can be converted into a combustible fuel such as methane. All that is needed to make this conversion is to replace the two oxygen atoms with four hydrogen atoms, resulting in CH4 (methane).
Researchers are working on turning captured CO2 into methane by using clusters of titanium oxide nanotubes coated with a catalyst that helps convert carbon dioxide and water into methane using sunlight as the power source.
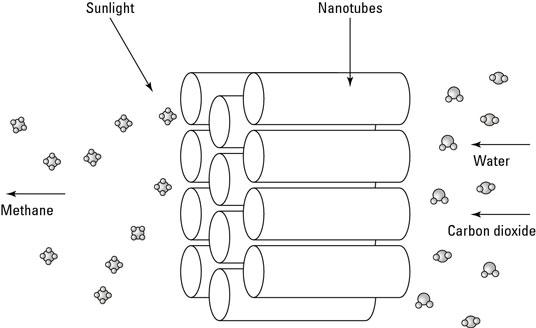
Because sunlight is used to convert the CO2, the additional energy costs in turning carbon dioxide back to fuel to power a plant wouldn't be considerable.
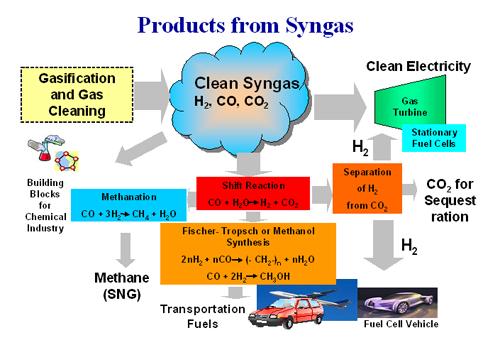
CO2 is a very stable, un-reactive molecule; but chemists can force it to react by pumping in electricity, heat, or both. The first step in this process is usually ripping off one of CO2's oxygen atoms to make CO. That CO can then be combined with H2 to make a combination known as syngas, which can be converted into methanol, a liquid alcohol that can be used as fuel. Massive chemical plants do just that, but they make their syngas not from air, but from plentiful and cheap natural gas.
Oil is inexpensive right now, so there is little incentive to adopt cutting-edge, costly alternatives. But the desire to halt climate change has drawn researchers around the world to the pursuit of CO2 conversion. However, there’s still a long way to go before the technology is ready for mass production, as it’s still too expensive.
Electric cars are slow to catch on, so we will not see an immediate change away from liquid fuel; and even if everyone purchased an electric car today, it will be around fifteen years before fossil fuel cars disappear as the main mode of transportation. Plus, collectors will keep these fuel guzzling vehicles on the road for decades.
The real challenge is going to be how do you make something like artificial photosynthesis at a reasonable scale and have it work in the real environment.

For additional information:
-
http://wordpress.ei.columbia.edu/lenfest/files/2012/11/SunlightToFuels_WhitePaper.pdf
-
https://www.netl.doe.gov/publications/proceedings/01/carbon_seq/7b1.pdf
Len Calderone - Contributing EditorLen contributes to this publication on a regular basis. Past articles can be found in the Article Library and his profile on our Associates Page He also writes short stores that always have a surprise ending. These can be found at http://www.smashwords.com/profile/view/Megalen. |
 |
The content & opinions in this article are the author’s and do not necessarily represent the views of AltEnergyMag
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
Featured Product


