All though there could be huge advantages to directly extracting carbon dioxide from our atmosphere instead of from its source, there has been very little R&D funding to explore and make it a reality. By beginning the process of recycling CO2, America would be building the technology now for a sustainable hydrocarbon future.
Len Calderone for | AltEnergyMag
Carbon dioxide (CO2) is a clear gas composed of one atom of carbon and two atoms of oxygen. Carbon dioxide has many chemical forms. Under standard temperature and pressure conditions, it is stable, inert, and non-toxic. Carbon dioxide occurs naturally in small amounts in the Earth's atmosphere.
Carbon dioxide helps create and maintain the natural greenhouse effect that keeps the Earth friendly to life. Without this effect, Earth’s temperature would be a chilly 0° F. Plants break down the CO2 into carbon and oxygen, releasing the oxygen in to the atmosphere, and retaining the carbon to live and grow.
Human activities are adding more CO2 to the atmosphere and affecting the ability of natural absorbers, such as forests, to remove CO2 from the atmosphere. While CO2 emissions come from natural sources, human activities are responsible for the increase that has occurred in the atmosphere since the industrial revolution began.
The combustion of fossil fuels, such as coal, natural gas, and oil for energy and transportation, are the chief sources of CO2 produced by humans. The increase in CO2 emissions is due to energy use by an expanding economy and population, along with emissions from electricity generation and the increase in vehicle travel.
Carbon dioxide emissions can be reduced by traveling in more fuel-efficient vehicles, and using more efficient appliances. We can also reduce CO2 emissions through conservation, such as reducing personal energy by turning off lights and electronics when not in use and driving fewer miles. We need to produce more energy from renewable sources and use lower carbon fuels. Another way to reduce CO2 emissions from coal and gas-fired power plants is carbon dioxide capture and sequestration.
Cutting back on CO2 emissions is not going to cut it. We need to start removing the CO2 that exists in the atmosphere now. Even if we stopped adding to the carbon dioxide that is presently in the air, this high level will continue for possibly thousands of years.
But how do we remove the excess CO2 from the air? Removing carbon dioxide from the air should not be confused with ongoing efforts to capture CO2 and sequestering it at its source, as I discussed in my last article, “Will Carbon Capture Reduce Global Warming.” That area is important, too, but it’s already being explored, and the technological demands are quite different.
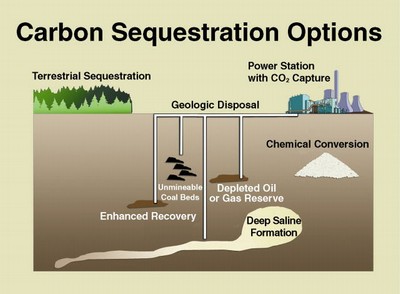
Extracting CO2 from the atmosphere is more difficult than extracting it from outlet gas, where the CO2 concentration is much greater.
Carbon dioxide is removed from the air by using a sorbent, then extracting the CO2 from the sorbent and sequestering it by pumping it deep underground at relatively high concentration or converting it back into fuel. Carbon dioxide can be taken directly from the atmosphere and turned into useful products like fuels and chemicals without having to go through the process of growing plants and extracting sugars, which can be fermented into fuels like ethanol. Plants can be removed from the equation.
One process uses a unique microorganism called pyrococcus furiosus, which thrives by feeding on carbohydrates in the super-heated ocean waters near geothermal vents. The organism's genetic material can be changed so that it can feed on carbon dioxide at much lower temperatures. Hydrogen gas is then used to create a chemical reaction in the microorganism that incorporates carbon dioxide into 3-hydroxypropionic acid, a common industrial chemical used to make acrylics and many other products.
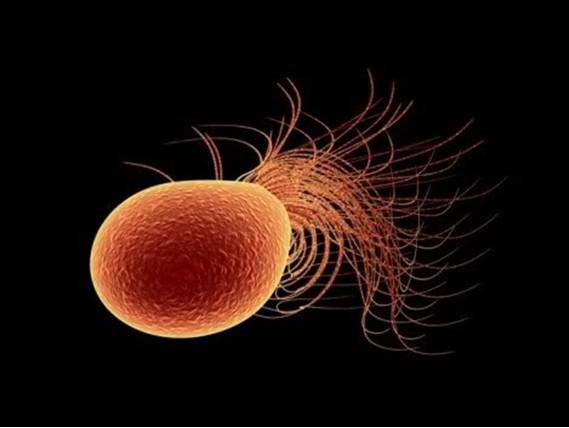
A single pyroccus furiosus bacterium
With the advent of carbon dioxide capture and sequestration, technologies for separating dilute carbon dioxide from various gas streams are gaining importance but most separation technologies are energy inefficient. At the Lenfest Center for Sustainable Energy, the range of applications for a recently developed moisture swing sorbent in the context of capturing CO2 from air has been extended. Moisture swing absorption has shown to have much greater efficiency for separating dilute carbon dioxide from various gas streams including the capture of carbon dioxide from the atmosphere.
The sorbent, an anionic exchange resin, has been shown to absorb CO2 when it is dry, and to release it again when exposed to moisture. In ambient air, the resin will dry again. After drying it is ready for another absorption cycle. In this way, the evaporation of water could actually be used to help reduce the energy problem associated with binding and subsequently extracting the CO2.
Super-absorbent fake leaves will be the answer to pulling a thousand times more carbon dioxide out of the atmosphere than trees, and this technology could be used to power our vehicles with green gasoline. This technology could be used to grow algae for biofuels, to make plastic, or used in soft drinks. The leaves look like sheets of papery plastic and are coated with a resin that contains sodium carbonate, which pulls carbon dioxide out of the air and stores it as bicarbonate on the leaf. To remove the carbon dioxide, the leaves are rinsed in water vapor and can dry naturally in the wind, soaking up more carbon dioxide.

An artificial "tree" used to capture carbon. (Photograph courtesy Klaus Lackner)
One such tree can remove one metric ton of carbon dioxide a day. By utilizing ten million of these trees we could remove 3.6 billion metric tons of carbon dioxide a year, or about 10% of the earth’s annual emissions. Total emissions could be removed with 100 million trees, which seem to be a lot, except that we would need 1,000 times that in real trees to have the same effect. Of course, storage would be a problem, but scientists are working on several solutions, such as sealing the absorbed gas as stable magnesium carbonate mineral, or pumping carbon dioxide into solidified gas bubbles from basalt formations from volcanic lava flows, causing it to react to produce stable limestone (calcium carbonate).
Of course, the best solution is to make fuel from the CO2 for our vehicles. Carbon dioxide can react with water to produce carbon monoxide and hydrogen, known as syngas because it can be readily turned into hydrocarbon fuels such as methanol or diesel. The process requires an energy input, but this could be provided by renewable sources.
All though there could be huge advantages to directly extracting carbon dioxide from our atmosphere instead of from its source, there has been very little R&D funding to explore and make it a reality. By beginning the process of recycling CO2, America would be building the technology now for a sustainable hydrocarbon future.
For additional information:
1. Capture of carbon dioxide from ambient air - K.S. Lackner
2. http://www.sv-jme.eu/data/upload/2012/05/01_2011_101_Pirc_04.pdf
3. http://www.netl.doe.gov/publications/proceedings/01/carbon_seq/7b1.pdf
About Len
Len has contributed articles to several publications. He also writes opinion editorials for a local newspaper. He is now retired.
This article contains statements of personal opinion and comments made in good faith in the interest of the public. You should confirm all statements with the manufacturer to verify the correctness of the statements.
The content & opinions in this article are the author’s and do not necessarily represent the views of AltEnergyMag
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
Featured Product